Use the activity series to predict the merchandise, if any, of every equation. Recognize chemical reactions because of single-replacement responses along with double-replacement reactions. You can easily buy different types of chemical products from echemi.
Use the table or the action series to predict whether every single replacement reaction will happen and, in that case, write a balanced chemical equation. Assuming that every single replacement reaction happens, predict the goods and compose every single balanced chemical formula.
Hydrosulfuric Acid
Then count the amount of each kind of atom within the equation that is perceptible. Explain the Use of the Law of Conservation of Mass at a chemical response. A chemical equation functions as a significant; communicative instrument for chemists. Between the reactants and the products, an arrow signal (⟶) is added to show how the response is happening.
1 dozen methane molecules along with 2 dozen oxygen molecules respond to yield 1 dozen carbon dioxide molecules along with 2 dozen fluids. From the aforementioned case between silver chloride, it's apparent that a purposeful net ionic equation could be written only when two electrons combine to produce an acidic substance. Write a balanced equation for the combustion of ethane C2H6 in air O2. The goods are carbon dioxide CO2 and water H2O. Write a balanced equation for the combustion of propane C3H8 in air O2. To be able to balance the chemical equation, then you want to be certain that the amount of atoms of each element on the reactant side is equivalent to the number of atoms of each element on the item side.
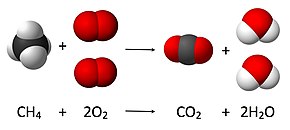
Occasionally'△' is written above the arrow to reveal that heating is required to cause chemical response. Chemical equation emblematic representation of a chemical response; reactants are reflected on the products and left to the right. Describe the symbols used to represent the conditions of things within a chemical formula.
Here is the Way to Activate The Chemistry Equation Automobile Formatter Plug
When a solid is formed in a response that happens in solution, it's called a precipitate. The creation of a precipitate is frequently indicated by underscoring. A molecule is the smallest part of a component which includes the chemical properties of the component. The atom of every component comprises the protons, neutrons, and electrons of the component.
In identifying substances, Greek prefixes have been utilized to signify the number of molecules present for every component. Compose the formulation and establish the stage for the merchandise and balance the following response. All chemical reactions are symbolized with compound equations. A makeup response generates one material from several reactants. The remaining polyatomic ions have adverse fees ranging from -1 to -4. Some common kinds are carbonate (CO32-), sulfate (SO42-), nitrate (NO3-), along chromate (CrO42-).
The yellowish color of this sulfur, within the form of a reactant, was absent from the item. Considering that the properties altered the item is new material. A chemical equation is a symbolic representation of each one of the compounds included in a chemical reaction. We utilize the chemical formulations of chemical to signify each compound species involved with the response. In addition, we use the notation or adhering to the compound formulation to recognize the stage of these substances from the equation. Within the following guide, we will speak about what type of chemical formula is, the way to balance chemical equations, and also provide you a few examples to assist in your balancing chemical equations clinic. In case the reactant or product is really a chemical then you have to understand how to write the formula of this chemical.
Write the net ionic equation for the reaction of Fe23 and Sr2. Write the net ionic equation for the reaction of KCl and NaC2H3O2.
Is Going to Be a Binary Covalent Compound. We Don't Understand What The
Write and balance the chemical equation explained by Exercise 4. A chemical formula is a succinct description of a chemical response. Write and balance the chemical compound which represents hydrogen and nitrogen responding to create ammonia, NH3.